Photocatalyst Activity Measuring Inks
There are many companies world-wide and operating in Europe who are currently promoting products that work via semiconductor photocatalysis. The function(s) of these semiconductor materials is/are usually based on one or more of the following three modes of action: photo-mineralization, photo-sterilization and photo-induced super hydrophilicity. As a result of the clear general usefulness of all of the above processes it is no wonder that a number of notable, different commercial products have arisen from the wide-scale research conducted in this area. These include: (i) self-cleaning glass, concrete, tent/awning materials and tiles, (ii) odour-removing paint for indoor applications, (iii) NOx removing paint, concrete and tiles for exterior applications, (iv) photo-induced sterile surfaces (ceramics and metals), (v) water and air purification units and (vi) defogging mirrors.
Such new materials and diverse commercial products require standards by which their effectiveness can be gauged, compared and contrasted. Standards help manufacturers to develop and deliver products which have the defined characteristics desired by their customers, such as photocatalytic activity, robustness, appearance and low cost. Thus, for industry, standards ensure their products are widely accepted and competitive, whereas, for the consumer they ensure product quality and reliability. It is appropriate, therefore, that the International Standards Organisation (ISO) have developed standards for photocatalysis. The European standards organisation (CEN) is now in the process of also creating standards for semiconductor photocatalytic products (CEN/TC386).
SMART INKS
The current (and planned) ISO and planned CEN tests of photocatalytic activity have three crucial drawbacks, namely, they: (i) are not rapid, (ii) require relatively expensive equipment and (iii) not suited for use in situ. The need for expensive equipment often also requires a dedicated technician to run and maintain it. The solution to this problem comes through the recent development of a range of rapid-acting, inexpensive photocatalyst activity-measuring inks (smart inks). A number of reports by different groups have established that these smart inks are able to identify and measure the photocatalytic activities of thin semiconductor films such as self-cleaning glass and tiles and yet, they have not so far been utilised as a possible route to creating a rapid, inexpensive and easy to use standard for assessing the activity of photocatalytic systems.
In such inks, an oxidised redox dye, Dox, such as resazurin (Rz), is mixed with a water-soluble polymer, such as hydroxyethylcellulose (HEC), a sacrificial electron donor (SED), most typically glycerol, and a solvent, usually water. The ink is cast on the surface of the photocatalyst (by spin-coating, doctor blade, printing or use of a felt-tipped pen) and when the photocatalyst is illuminated with ultra-bandgap light, the photogenerated hole oxidises the SED whilst the photogenerated electrons reduce the dye to its differently-coloured, reduced form, Dred; thus, the SED essentially acts as a hole-trap to prevent electron-hole recombination. A schematic illustration of the major steps involved in such a typical photocatalyst indicator ink is shown in figure below.

Schematic illustration of the mechanism by which a typical photocatalyst indicator ink works. Thus, following illumination of the TiO2 photocatalyst by ultra-bandgap light, an electron-hole pair (e-, h+) is produced. The hole oxidises the SED, e.g. glycerol, to SEDox, e.g. glyceraldehyde and/or glyceric acid. The electron reduces the dye from its oxidised form, Dox, to its reduced counterpart, Dred (step 1). If Dred is oxygen sensitive, its undesirable oxidation (from Dred to Dox) can occur (step 2).
In the case of the Rz photocatalyst indicator ink, Rz is Dox, Dred is resorufin (Rf), and the reduction process is accompanied by a colour change from blue to pink. Since the reduction of Rz to Rf is an irreversible reaction, Rf cannot be re-oxidised to Rz, i.e. step 2 in figure 1 is not possible. The process is rapid, in that the dye reduction is a two electron step and the photo-oxidation of glycerol is very efficient, whereas in the more traditional photocatalytic oxidation process (such as in the ISO and CEN standards that use the photobleaching of methylene blue or rhodamine b), the dyes are difficult to photobleach and their complete mineralisation requires many photogenerated holes (204 for methylene blue for example).
For a Rz-based, photocatalyst indicator ink the typically blue to pink colour change, signifying the completion of the photoreduction reaction, is achieved in less than 6 minutes even for very thin (ca. 15 nm) commercial films of titania, such as Pilkington Glass ActivTM under a moderate UVA illumination irradiance of ~ 3 mW/cm2.
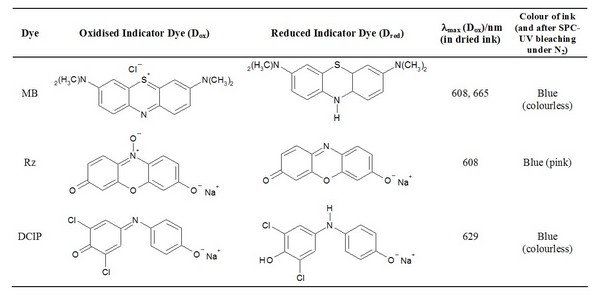
Table 1: Structures and properties of redox dyes used in photocatalyst activity indicating inks.
Following on from this work several other photocatalyst indicator inks have been developed, including ones based on dichlorophenol (DCIP) and methylene blue (MB). The structures of these dyes and their main visible spectral characteristics are given in table 1. The ready, apparently irreversible, photocatalysed reduction (note NOT oxidation) of methylene blue is effected by making the pH of the ink very low (pH), since under such conditions the reduced (leuco) form of the dye is not very air-sensitive. Each of these inks have been tried and tested and shown to work very effectively on commercial photocatalytic samples. Table 2 below indicates the apparent activities of each of these inks.
Dye |
Half life (t½) |
Resazurin |
132 s |
2,6-dichloroindophenol |
56 s |
Methylene blue (~ pH 2) |
17 s |
Table 2: Observed photocatalyzed bleaching (in air) half-lives of the natural DCIP and RZ inks and an acid MB ink, deposited onto ActivTM; incident UV-A intensity 3 mW/cm2.
The results in table 2 are the observed times for the inks to change colour and necessarily imply that there is no need for a skilled operator or use of expensive analytical equipment to monitor reaction progress, as the naked eye is sufficient when using these inks for a qualitative indicator, and a semi-quantitative assessment, of photocatalytic activity. Additional work shows that the initial rate of the Rz to Rf colour change is directly related to the photocatalytic activity of the titania-coated glass, as measured by the stearic acid test or dye (usually Acid Orange II) photo-oxidation tests, i.e. the rate of dye colour change is directly related the PCO activity. Finally, photocatalyst indicator inks are inexpensive, fast-acting and readily interpreted. The incorporation of such inks into a standard for photocatalysis will address the significant industrial need for fast, easy to use effective method for assessing the photocatalytic activities of most products in the lab and in situ. Such a standard will not only provide greater QC but also help significantly with the marketing of such products.

